Background: Myelodysplastic syndromes (MDS)-related anemia leads to significant morbidity, due to impaired oxygen transport and red blood cell transfusion (RBCT)-related iron overload. Erythropoiesis-stimulating agents (ESAs) are used to treat anemia and decrease transfusion burden and are effective in ~ 35% of patients (pts) with lower-risk (LR)-MDS. Luspatercept is approved for pts with LR-MDS refractory/intolerant to ESAs who need regular RBCTs. Primary resistance to ESAs is frequent, and ~ 70% of responders relapse in < 2 years. Baseline (BL) erythropoietin level ≤ 200 U/L and ≤ 2 somatic mutations are prognostic factors for better ESA response. An association of driver gene mutations with deteriorating clinical outcomes is well established in LR-MDS (Papaemmanuil E, et al. Blood 2013;122:3616-3627; Bersanelli M, et al. J Clin Oncol 2021;39:1223-1233; Nazha A, et al. J Clin Oncol 2021;39:3737-3746). The Molecular International Prognostic Scoring System (IPSS-M; Bernard E, et al. NEJM Evidence 2022;1(7):EVIDoa2200008) was developed to improve upon the shortcomings of the Revised IPSS (IPSS-R) and restratified ~ 46% of pts by adding genomic profiling to existing hematologic and cytogenetic parameters. Pts with LR-MDS typically harbor a median of 2-3 driver mutations in genes involved in MDS pathogenesis.
Methods: Individual pt bone marrow samples were prepared for sequencing using the Illumina ® DNA prep with enrichment and the xGen™ hybridization capture of DNA libraries. Exon coverage was ≥ 400X for 3% sensitivity. Sequencing libraries were run on the NovaSeq6000 (Illumina ®) system and statistical analyses were conducted using R. A targeted panel of 82 genes was used.
Results: At BL, 322/363 pts enrolled in the COMMANDS trial had somatic mutations in ≥ 1 gene; 11 pts did not have mutation data. Most mutations had a variant allele frequency of 3%-50%. In the intent-to-treat (ITT) population, the luspatercept arm did not show any significant differences in BL mutational burden (MB, defined as the number of gene mutations from the myeloid gene panel) between responders (R) and non-responders (NR). Interestingly, R in the ESA arm trended ( P = 0.065) towards lower MB compared with NR. Subgroup analysis revealed that ring sideroblast (RS)− pts in the ESA arm had lower MB at BL (median 1 gene mutation) compared with RS+ pts (2 gene mutations; P = 0.0004). However, no significant difference was observed in the luspatercept arm.
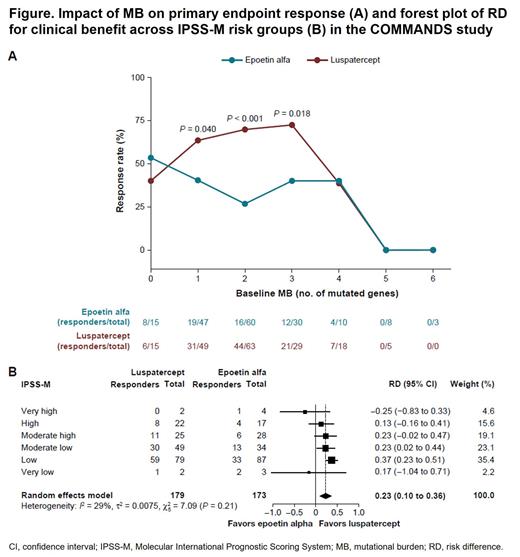
In the ITT population (and the RS+ subgroup), compared with ESAs, luspatercept showed robust responses (Figure A) in pts with 1 (63% vs 40%; Fisher's P = 0.040), 2 (70% vs 27%; Fisher's P < 0.001), and 3 (72% vs 40%; Fisher's P = 0.018) gene mutations. However, in the RS− subgroup, luspatercept and ESAs had similar response rates independent of MB. Interestingly, more RS− pts in the luspatercept arm had ≥ 4 gene mutations (12/47 pts [26%]) versus the ESA arm (only 3 pts [6.4%]). Furthermore, these ≥ 4 mutations were in genes associated with shorter leukemia-free survival, overall survival, and progression-free survival. Of the RS− pts in the luspatercept arm, 9/12 were NR, compared to 2/3 in the ESA arm.
Luspatercept showed superior clinical benefit in pts with difficult-to-treat mutations (such as ASXL1, TET2 DNMT3A ZRSR2) and favorability in pts with mutations in SRSF2 EZH2, TP53, DTA.SF3B1.n and IDH2. Primary endpoint analysis by IPSS-M (Figure B) also showed superior clinical benefit of luspatercept across risk groups compared with ESAs: Low (75% vs 38%); Moderate low (61% vs 38%); Moderate high (44% vs 21%); and High (36% vs 24%).
Conclusion: In pts in the COMMANDS trial,BL MB impacted response to ESAs but not to luspatercept. In both ITT and RS+ pts, luspatercept showed either superior or comparable clinical benefit in pts with LR-MDS with various BL MB. Interestingly, both luspatercept and ESAs had similar clinical benefit across various MB in the RS− subgroup. However, lower MB favored higher responses to ESAs in the RS− subgroup, and higher MB in genes associated with poor prognosis significantly impacted response rates in the luspatercept arm. Despite the presence of these imbalances in BL MB, RS− pts had similar response rates to luspatercept as to ESAs. Pts with LR-MDS with difficult-to-treat mutations also showed superior or favorable clinical benefit with luspatercept. Finally, luspatercept showed superior clinical benefit to ESAs in pts with LR-MDS across all IPSS-M risk groups.
Disclosures
Komrokji:AbbVie, CTI biopharma, Jazz, Pharma Essentia, Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; Rigel, Taiho, DSI: Honoraria, Membership on an entity's Board of Directors or advisory committees. Guerrero:Bristol Myers Squibb: Current Employment. Garcia-Manero:Genentech: Research Funding; AbbVie: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding. Zeidan:Astellas: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Shattuck Labs: Research Funding; Astex: Research Funding; Seattle Genetics: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Ionis: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Mendus: Consultancy, Honoraria. Platzbecker:MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Research Funding; Celgene: Honoraria; BMS: Research Funding; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Roche: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Geron: Consultancy, Research Funding; AbbVie: Consultancy; Fibrogen: Research Funding; Merck: Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; BeiGene: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Janssen Biotech: Consultancy, Research Funding; Syros: Consultancy, Honoraria, Research Funding. Hayati:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Vodala:Bristol Myers Squibb: Current Employment; Mabgenex: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal